For many industries and organizations around the world, ISO 9001 has evolved to become the foundational quality standard on which their complete quality management system is built.
However, as the integration of technology has changed many aspects of systems and operations in recent years, a degree of inflexibility and rigidness in the current ISO 9001:2008 standard was exposed. This latest revision-due to be published in September, 2015-will allow organizations to be more flexible in how they apply the standards and practices of IS0 9001 in the context of their unique processes, technologies and business needs.
The following questions and answers have been chosen for inclusion in this FAQ and organized by topic to facilitate organizations’ understanding of the changes afoot and how they will impact their business.
Where can I learn about the new structure of ISO 9001:2015?
Quality Resource Center has produced an abundance of materials that include overviews and analysis of the new high-level structure of ISO 9001, Annex SL. Quality Resource Center offers a full suite of value added training, including on-site, web based, as well are our state of the art training facility in northern California. The materials are available on the website.
You may also contact Quality Resource Center with any questions at certification through our web portal or by simply calling 1 800 244 5409.
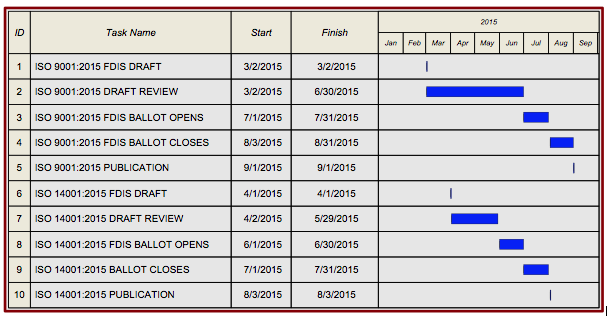
When will the final version of ISO 9001:2015 be published, and where can I get a copy?
Timeline for publication and implementation of ISO 9001:2015
- July- October 2014: Comments on DIS were submitted
- July 2015 Final Draft International Standard FDIS issued
- September 2015 (estimate): ISO 9001:2015 issued
- September 2015- September 2018: Transition period. All current registrations to ISO 9001:2008 must be transitioned to the 2015 revision by this time, or they will lapse.
Will there be an implementation guide published for ISO 9001:2015?
Yes. Quality Resource Center will continue to offer additional important guides and updates. We also provide ISO 9001:2015 consulting, training, and auditing for businesses in need.
When should I start transitioning to ISO 9001:2015, and what should I do to prepare?
It would be prudent to begin planning discussions now based on the DIS, but hold off on taking any major action until the standard is published and the transition officially begins in September, 2015. One thing you can do is start taking inventory of your current processes and comparing them to the new high level structure proposed in the Dl S.
How much longer will ISO 9001:2008 compliance be recognized?
Conformance to the current standard will be recognized through the end of the three-year transition period, which is due to end September, 2018. Organizations can request to be audited to the FDIS when it is issued. The issuance of certificates is an Assurance Group decision. All organizations must transit ion to the new standard by the end of the transition period.
Are Organizations Permitted to Upgrade During Their Scheduled Re-certifications in 2016?
Yes, as long as your systems conform to the standards set forth in ISO 9001:2015.
Our Organization is Currently Implementing or Considering Certification to ISO 9001:2008. What is the Best Course of Action?
Continue as planned; there are three full years to achieve certification to ISO 9001:2015 after its publication in September, 2015. That said, Quality Resource Center can help to familiarize Organizations with the new high-level structure, so systems designed or upgraded with an eye toward the future.
Will the Transition Require Additional Resources Either in Budgets or Time?
Quite likely, but it will depend upon the current status of an Organization’s management system. Areas that will be impacted include personnel time developing or modifying current systems and processes to meet the new requirements.
For more information regarding how the ISO 9001:2015 might impact your organization, Quality Resource Center offer detailed ISO 9001:2015 GAP Analysis services that can assist in identifying where the audit against the revised standard will differ from your existing programme.
When May I begin transitioning? Do I Need to Wait Until My Surveillance Audit?
The transition process may commence as soon as the official three-year transition begins. While it is permissible to outside of your scheduled surveillance but it is more efficient to do an upgrade audit to ISO 9001:2015 during regularly scheduled surveillance audits.
How can Progress Best Be Measured During the Transition Process?
Quality Resource Center works closely with Organizations to develop approaches enable progress to be tracked towards the new standard and ensure proper training, support, and execution every step of the way. If you are implementing the standard for the first time, Quality Resource Center offers full turn-key solutions that offer maximum value with minimum risk.
When will auditor training be available for ISO 9001:2015?
Internal auditor training and certification will be available following publication of the FDIS in 2015; the rules and requirements have not yet been finalized. If your organization has a robust Internal Audit process, the auditing techniques should not change. However, the criteria you audit against and the scope of your audits will definitely be affected.
Quality Resource Center will provide customers with additional information as it becomes available. We also have a series of interactive webinars already scheduled, and public training courses on the transition all over North America. We also have a series of interactive webinars already scheduled, and public training courses on the transition a II over North America.
ISO 14001 Is Being Revised Along With ISO 9001. Will the Impact Organizations Utilizing Both Standards?
ISO 9001:2015 and ISO 14001:2015 will be more closely aligned than ever, as both standards will utilize the same high-level structure defined in Annex SL. Upwards of 30% of the language in the two management system standards will be identical. ISO 14001:2015 should be issued in September 2015 along with ISO 9001:2015 if everything goes according to plan.
What Impact Will the Revisions Have On ISO/TS 16949:2009 Automotive Standard?
The automotive industry is not enamored with the revisions to ISO 9001. Currently they are opting out of participating in the revision process in favor of creating their own standard. While the automotive group could certainly change their position between now and when the standard is finally issued in September 2015, no definitive position has been determined.
We Are Certified to AS 9100C. Are there any plans to revise this standard to accommodate new ISO 9001:2015 requirements?
AS 9100 will adopt the requirements of ISO 9001:2015 when the new standard is revised in 2016.
Is the ISO 13485 Medical Device Standard Changing As A Result Of The Updates To ISO 9001?
The draft ISO 13485:2015 is based on the clause structure in ISO 9001:2008, not the new requirements and clause structure of ISO 9001:2015 using Annex SL.
Tables correlating ISO 9001 and the previous version of ISO 13485 have been included in the document.
Similar to ISO 9001:2015, there will be a transition period for organizations upgrading from the 2003 revision to the ISO 13485:2015 standard. The date of the ISO 13485 transition has not been announced as yet.
The supplementary Annex Z in support of the European Medical Devices Directives has been included in anticipation of the next revision of ISO 13485 being harmonized under the three Medical Device Directives.
This means ISO 13485 and EN ISO 13485 will be published in a similar timeframe. Organizations can then use the Harmonized Standard with in Europe which provides for the “Presumption of Conformity” under the applicable clauses of the Directives. This revision has also been drafted recognizing that it will have to support the existing European Medical Devices Directives and the proposed European Medical Device Regulations when they are published in the future. Some of the content has therefore been specifically drafted to accommodate this requirement.
Where does Plan-Do-Check-Act (PDCA) Fit In With The New Standard?
Of course. The new standard is still built around the PDCA cycle. It’s featured prominently on page 8 of the Draft International Standard.
Will FMEA’s and Control Plans be required under ISO 9001:2015?
Although very valuable tools, FMEA’s and Control Plans are not required by ISO 9001:2015. Your organization could apply these tools as their approach to meet the requirements. Properly developed Control Plans would satisfy many of the requirements for section 8. FMEA’s are a useful tool to identify prioritize, and mitigate areas of risk required in section 6.
What Is The Practical Impact Of Eliminating The Management Representative?
The management system still needs a champion and a spokesperson, but it does not automatically mean that this is the quality manager-or any one person. Optimally, these duties would be shared by a member(s) of the leadership team. Once the management system has been implemented, this person’s role should transform into being a facilitator of continual improvement. It should be noted that while the requirement for a designated Management Representative has been removed from the DIS, the responsibilities that were attributed to this position are still retained in the standard.
What Suggestions Are There For Organizations Wishing To Begin Preparing For ISO 9001:2015?
- Have a GAP Analysis performed and review the results. Identify Actions to fill the GAP’s.
- Train managers that will be affected by the proposed changes. Help them gain a comprehensive understanding of the issues at hand and help them begin to strategize an action plan for implementation
- If you have certification to more than one standard, look at where management system integration might be possible and beneficial. Make this part of the GAP Analysis.
- Talk to one of our representatives and ask how we can support your organization
I have an integrated management system based on ISO 14001 and OHSAS 18001-how will the revisions to ISO 9001 and these standards affect me?
The changes make system integration much easier as there will be greater alignment among these standards. But with differing projected publication dates and trans-national, you should plan your transitions carefully to retain certification on each.
It may be beneficial to acquire a copy of PAS 99, It offers valuable guidance on the design and structure of an integrated management system.
Is There A Requirement For a Quality Manual in ISO 9001:2015?
ISO 9001:2015 does not require a quality manual. The question each organization should consider is whether or not having one is beneficial to the organization. If it is used as intended in 9001:2008-an introduction to the management system that acts as a guide or roadmap to the overall system-then by all means have one. Quality Resource Center strongly recommends the Organizations maintain a Quality Manual.
What Are The Guidelines Like For Internal Audits Under ISO 9001:2015?
Refer to section 9.2.2 Internal Audit –
9.2.2
“The organization shall:
- a) plan, establish, implement and maintain an audit programme(s) including the frequency, methods, responsibilities, planning requirements and reporting, which shall take into consideration the quality objectives, the importance of the processes concerned, customer feedback, changes impacting on the organization, and the results of previous audits;
- b) define the audit criteria and scope for each audit
- c) select auditors and conduct audits to ensure objectivity and the impartiality of the audit process”
These requirements have been clarified from those in 9001:2008. Audits are still required to be conducted at planned (scheduled) intervals. Organizations need to establish goals, priorities, and objectives and align them to drive their decisions with respect to the audit function. Many of factors go into the development of an audit program, risk is being only one of them. Other things to consider: complexity and current state of the process or areas involved, and potential impact on customers and the Organization.
What Are The Key to Requirements for Design and Development of Products and Services? Will there be Requirements for Process Design?
Much of what is now required under ISO 9001:2015was implied and would have been considered good practices under ISO 9001:2008. Section 8.3 “Design and Development of Products and Services” enhances the requirements found in section 7.3 of ISO 9001:2008, which dealt with product design. Some of the requirements have been reorganized (i.e. verification and validation have been consolidated in one section 8.3.4 “Design and Development Controls”). Technically, manufacturing process design is not included. However, a note at the end of section 8.3.1 encourages organizations to apply these same principles to process design and development.
Can you summarize the new approach to Preventative Action in ISO 9001:2015?
Organizations must understand their organization’s business context (Clause 4.1) and determine the risks and opportunities that need to be addressed (Clause 6.1). One of the key purposes of a quality management system is to act as a preventive tool. Consequently, the new standard does not have a separate clause or sub-clause titled “Preventive Action.” Instead, the concept of preventive action is expressed through a risk-based approach to formulating quality management system requirements. This resulted in a reduction of prescriptive requirements, which have been replaced by performance-based requirements. Although risks and opportunities have to be determined and addressed, there is no requirement for formal risk management or a documented risk management process. The implication is the entire management system, properly implemented, should function as a preventive tool.
Does Compliance With ISO 31000 Satisfy The Risk Management Requirements in ISO 9001:2015?
Clearly an Organization could apply the requirements of a risk management system as defined in ISO 31000 and more than meet the requirements for risk based thinking in ISO 9001:2015. While the standard does not require a formal risk management approach, organizations must identify and understand their business environment in the broadest of terms and the resulting potential risks they face. Armed with this data the organization can then develop and deploy an effective management system to control, mitigate, and eliminate these risks.
Is there a summary of the changes between ISO 9001:2008 and 9001:2015 available?
Yes. Quality Resource Center provides this summary and has made it discuss it at our training courses, both classroom and interactive webinars
How can Quality Resource Center provide support through the transition process?
Quality Resource Center will have the latest up-to-date information on the ISO 9001 revision all the way up to the planned publication in September, 2015. Upon publication, we can advise you on what to do to meet the new requirements.
Ultimately, it is up to you to plan and implement the changes, but Quality Resource Center will provide all the support you need to make the best possible decisions.
What training will be available?
Yes, we have a series of interactive webinars already scheduled, and public training courses on the transition all over North America.
I have questions about my certification I need to answer right now. Who should I call?
Quality Resource Center – 1 (800) 244 5409 or e-mail us at www.QRCCentral.com